Afanat 30mg Tablet
- Estimated Delivery : Up to 4 business days
- Free Shipping & Returns : On all orders over $200
Brand Name: Afanat 30mg Tablet |
|---|
Product Name: Afatinib Dimaleate |
|---|
| Package Size : each bottle contain 28 tablets |
|---|
Afanat 30mg Tablet (Afatinib Dimaleate) is an irreversible ErbB family receptor inhibitor approved for treating epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC). Composition: Each tablet contains 30mg Afatinib dimaleate with pharmaceutical excipients ensuring bioavailability and stability. Indications: Afanat is first-line therapy for NSCLC harboring EGFR exon 19 deletions or exon 21 (L858R) substitution mutations; treatment of metastatic NSCLC with EGFR mutations; potential use in HER2-mutant lung cancer. Clinical efficacy demonstrates significantly improved progression-free survival (13-15 months) compared to chemotherapy in mutation-positive patients. Contraindications: Absolute contraindications include hypersensitivity to afatinib components, severe hepatic impairment, and concurrent use of strong P-gp inhibitors. Relative caution required with baseline respiratory disease, cardiac dysfunction, or GI complications. Drug Interactions: Major interactions with P-glycoprotein inhibitors (erythromycin, clarithromycin – increases afatinib levels), P-gp inducers (rifampin reduces levels), strong CYP3A4 inhibitors, and antacids (separate dosing by 2 hours). Dosage & Administration: Standard dosing is 40mg once daily; can escalate to 50mg if tolerated or reduce to 30-20mg for toxicity management. Take on empty stomach 1 hour before or 2 hours after meals. Storage: 15-30°C in original moisture-protective packaging away from light. Side Effects: Common: diarrhea (90% incidence requiring antidiarrheal management), rash/acneiform dermatitis (80%), stomatitis, nail toxicity, fatigue. Serious: pneumonitis (3-4%), hepatotoxicity, ventricular dysfunction, severe diarrhea-induced dehydration. Precautions & Warnings: Weekly LFTs first month; baseline and periodic ECG; aggressive diarrhea prophylaxis with loperamide; close monitoring for interstitial lung disease signs; infection screening essential. Pregnancy & Lactation: Afatinib is teratogenic (Category D); absolutely contraindicated in pregnancy. Males require barrier contraception during therapy. Females require hormonal contraception plus barrier methods with monthly pregnancy tests. Breastfeeding contraindicated due to potential harm. Substitutes & Alternatives: First-generation EGFR TKIs (Gefitinib, Erlotinib) for EGFR-mutant NSCLC; second/third-generation agents (Osimertinib, Dacomitinib) for T790M-resistant disease. Chemotherapy (Pemetrexed-Cisplatin) for wild-type EGFR patients. FAQs: Expected response time 4-8 weeks; diarrhea management strategies (dietary modification, loperamide, budesonide enema); cost and insurance coverage varies; treatment duration until progression or intolerance. Expected Response Timeline: Week 4 clinical assessment; Week 8 imaging evaluation (CT chest); Month 3 response confirmation via RECIST criteria; ongoing monthly surveillance. Book Thoracic Oncology Consultation: Essential for EGFR mutation testing coordination, toxicity management strategies, TKI selection optimization, and disease monitoring protocols. Schedule Laboratory Tests: Baseline CBC, LFTs, renal function; weekly LFTs x 4 weeks then monthly; baseline ECG, troponin; monthly CT chest/abdomen; monthly EGFR mutation and ALK rearrangement testing; weekly diarrhea assessment and management.
Only logged in customers who have purchased this product may write a review.


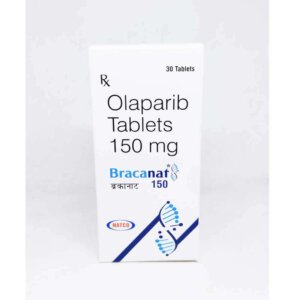





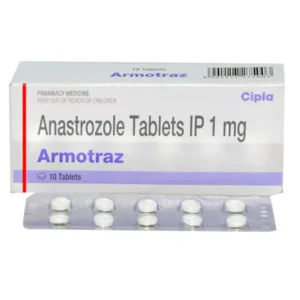



 Rebopag 50mg Tablet
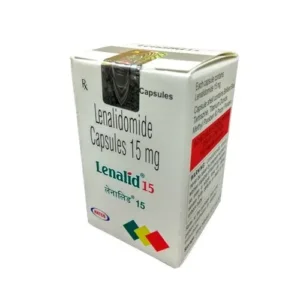
Rebopag 50mg Tablet  Lenalid 25mg Capsule
Lenalid 25mg Capsule  Daclahep 60mg Tablet
Daclahep 60mg Tablet 

Reviews
There are no reviews yet.